作者简介:王晓龙,1983年生,男,博士,高级工程师。
通过水热晶化法,合成含有骨架杂原子Zn的Zn-HZSM-5分子筛催化剂,采用XRD、BET、NH3-TPD表征催化剂结构和物化性能,在微型固定床反应器中测定催化剂的甲醇制汽油反应性能和反应动力学数据,研究Zn-HZSM-5分子筛催化剂的甲醇制汽油本征反应动力学。结果表明,杂原子Zn引入ZSM-5分子筛骨架后增加对产物汽油选择性有利的弱酸量。采用Chen and Reagan建立的甲醇制汽油三集总动力学模型,通过四阶龙格库塔法和最小二乘法对实验数据的回归,计算反应速率常数为 k1=1.154×1012exp (-97600/R T), k2=0.687×1012exp (-105200/R T)和 k3=1.739×107exp (-84700/R T)。以目标残差函数OF参数值为检验模型的标准,模拟值和实验值的相关系数 R2均在0.993以上。因此Chen and Reagan建立的甲醇制汽油三集总动力学模型可以准确描述Zn-HZSM-5分子筛催化剂的甲醇制汽油反应动力学行为。
Zn-HZSM-5 zeolite catalysts containing skeleton heteroatom Zn were synthesized by in-situ hydrothermal crystallization method.Structure and physic-chemical properties of the catalyst were characterized by XRD,BET and NH3-TPD.The catalytic activity and intrinsic reaction kinetics for methanol to gasoline of Zn-HZSM-5 zeolite were measured in micro fixed bed reactor.Results showed that amount of weak acid beneficial for gasoline selectivity increased when heteroatom Zn was modified into ZSM-5 zeolite skeleton.Using the three-lumped kinetic model of methanol to gasoline established by Chen and Reagan,the reaction rate constants were k1=1.154×1012exp (-97600/R T), k2=0.687×1012exp (-105200/R T)and k3=1.739×107exp (-84700/R T) respectively based on experimental data regression by Runge-Kutta method and the least-squares method.Taking the target residual function (OF) parameter value as criterion of test,the correlation coefficients R2 of simulated value and experimental value were higher than 0.993.Therefore,the methanol to gasoline three-lumped kinetic model established by Chen and Reagan could accurately describe the kinetics of methanol to gasoline reaction on Zn-HZSM-5 zeolite catalysts.
In recent years, the demand for oil in China has been growing larger and larger, which has led to the increasing dependenceon foreign oil. It is estimated that China's dependence on foreign oil may be exceed 65% by 2020, posing a huge threat to China's energy security. Coal reserves in China's fossil fuels far exceed the reserves of oil and natural gas. The development of coal-based methanol to gasoline (MTG) can reduce the dependence on foreign oil and increase the value of coal chemical industry, which is very important for the healthy and sustainable development of coal chemical industry[1, 2]. Compared with gasoline produced from traditional petrochemical industry, gasoline produced from coal-based methanol has advantages of high octane number, good explosion-proof property, no sulfur and chlorine[3, 4].
ZSM-5 zeolite catalyst for methanol to gasoline process has high activity, good selectivity and long life[5]. ZSM-5 zeolite catalyst has relatively strong acid, which leads to deep cracking and decreases gasoline yield. Therefore, transition metals are usually used to modify ZSM-5 zeolite catalyst to reduce acidity[6, 7]. Ni Y et al[8]introduced Zn metal onto ZSM-5 zeolite and found that synergistic effect between Zn and zeolite acidic sites improved selectivity of hydrocarbons above C6.Bi Y et al[9]in-situ synthesized ZSM-5 zeolite containing transition metal Zn, Zn played a regulatory role on ratio of B acid and L acid, and reduced strong acid while enhancing weak acid content. Ni Y et al[10]studied the catalytic activity of Zn-ZSM-5 zeolite for methanol to aromatics. It was found that selectivity of aromatics increased and carbon deposition rate decreased significantly. Before industrial application of Zn-HZSM-5 zeolite in methanol to gasoline, it is necessary to acquire intrinsic kinetics of Zn-HZSM-5 zeolite to guide the modification of MTG reactor and optimize reaction conditions.
In thekinetic model based on mechanism of methanol production reaction, all kinds of reaction types and all elementary reactions and intermediate species are taken into account through quantum method[11]. It is very difficult to widely used in industrialization studies because the regression of kinetic parameters is extremely difficult due to the multiplicity of elementary reactions and reaction species. Based on the hydrocarbon pool reaction mechanism[12], Kumar P et al[13]studied catalytic methanol production kinetics over ZSM-5 zeolite in fixed bed reactor. A mechanistic kinetic model containing 294 elementary reactions and 91 intermediate reaction products was constructed. All elementary reactions such as isomerization reaction, oligomerization reaction, alkylation reaction, oligomerization and methylation reaction were considered, making the regression of kinetic parameters very difficult. The lumped dynamic model[14, 15, 16] simplifies the complex reaction network into several typical reaction products and reaction paths. References[17, 18] studied the kinetics of MTG using an integrated fixed-bed reactor. The lumped kinetic model established by Chen and Reagan was used to simplify the reaction product into three components:oxygenated compounds(methanol and dimethyl ether), light olefins(ethylene and propylene) and gasoline components. The lumped kinetic model can describe kinetic behavior of ZSM-5 zeolite well.Based on the carbene reaction mechanism[19], Soltanali S et al[20] studied the kinetic behavior of MTG catalyzed by HZSM-5 zeolites with different particle sizes using the kinetic model established by Chen and Reagan[21]. The results showed that HZSM-5 zeolite catalyst of large size contained more surface acidic sites and was more suitable for MTG reaction three-lumps kinetic model.
In this paper, Zn-HZSM-5 zeolite catalyst was prepared by in-situ synthesis method. Physical properties of Zn-HZSM-5 zeolite catalysts were characterized.In a fixed-bed micro-reactor, the methanol to gasoline activity of Zn-HZSM-5 zeolite catalyst was evaluated. After elimination of internal and external diffusion, intrinsic kinetics of methanol to gasoline reaction over Zn-HZSM-5 zeolite catalyst was studied. Based on the carbene reaction mechanism, the three-lumped kinetic model established by Chen and Reagan was used to calculate the intrinsic kinetic data obtained though orthogonal experiments by four-order Runge-Kutta and least squares methods. Finally, the three lumped kinetic model parameters were calculated and the kinetic parameters were verified.
Zn-HZSM-5 zeolite catalystwas synthesized in-situ by hydrothermal method[22]. According to a certain ratio, TPAOH, sodium hydroxide and silica sol (mass fraction of 30%) mixed in deionized water to get Solution A. Sodium aluminate and different quality of zinc nitrate were added into deionized water to get solution B. Under stirring, solution B was slowly added dropwise to solution A. After the dropwise addition was completed, pH of the mixed solution was adjusted to 10.5 using sodium hydroxide solution of certain concentration or dilute sulfuric acid solution. The mixed solution was stirred at room temperature for 2 h, then moved into reactor. The prepared gel was crystallized statically at 413 K for 24 h. The solid product was filtered and washed with deionized water until the pH of the washing water reached 7-8 and then dried at 393 K for 12 h. The final product was calcinated at 823 K for 5 h. A certain amount of ammonium chloride solution with a mass fraction of 10% was arranged, the ammonium chloride solution mixed with the original powder of ZSM-5 zeolite with different mass contents of Zn in a mass ratio of 10 : 1 and stirred in water bath at 353 K for 5 h. The slurry was washed and filtered. Then the operation was repeated twice. Finally, the residue was dried and roasted to get HZSM-5 zeolite catalyst containing different content of skeleton Zn.Through XRF characterization for Zn-HZSM-5 zeolite catalyst, Zn content ranged from mass fraction of 0.90% to 3.69%. The synthesized Zn-HZSM-5 catalyst was compressed and sieved to evaluate activity of MTG. In order to compare with Zn-HZSM-5 zeolite catalyst, ZSM-5 zeolite was synthesized by hydrothermal method without adding zinc nitrate. Under the same ratio of silica to alumina, HZSM-5 zeolite catalyst was obtained.
Crystallization of zeolite catalysts were characterized by X-ray powder diffraction analysis (XRD) using Japan Rigaku company D/max-2500 instrument with Cu radiation(y = 0.154 056 nm). Working voltage was 40 kV and current was 40 mA. Scan speed was 5° · min-1, scanning step was 0.02° , scanning angle was 5° -45° .
Specific surface area, pore volume and pore structure of zeolite catalyst were tested by nitrogen-physisorption characterization using the United States Quadrasorb SI-KR.
NH3-TPD characterization was carried out to determin acid type and acidity of the zeolite catalyst using the United States Mieromeritics company Autochem II 2920.
The micro-fixed bed reactor was used to test catalyst activity. A certain amount of Zn-HZSM-5 zeolite catalystwas put into the reaction tube constant temperature section, the rest of the reaction tube filled with quartz sand. Under nitrogen atmosphere and 1 MPa, the reactor temperature was raised to a certain temperature. Then, under the conditions of activation, Zn-HZSM-5 zeolite catalyst was activated. After that, methanol was driven into the preheater through a parallel flow pump to be gasified and reacted on Zn-HZSM-5 zeolite catalyst. High-temperature hydrocarbon products were first condensed and then subjected to gas-liquid separation. Gas phase product and collected liquid product were then analyzed by Agilent GC 7890B gas chromatography with hydrogen flame ionization detector using PONA software. Yield and selectivity of each hydrocarbon was calculated based on carbon balance. Gasoline component contained all C5-C11 hydrocarbons.
Effect of internal diffusion is eliminated by changing particle size of Zn-HZSM-5 zeolite catalyst while keeping all other reaction conditions constant. Under the same reaction temperature, reaction pressure, space time and catalyst loading, only particle size of Zn-HZSM-5 zeolite catalyst changes. Methanol conversion is stable when particle size of zeolite catalyst decreases to a certain value. When particle diameter is less than or equal to this, influence of internal diffusion has been eliminated. Effect of external diffusion is eliminated by changing the amount of Zn-HZSM-5 zeolite catalyst in reactor tube and feed of raw materials to ensure that their ratio does not change. When the linear velocity of gas stream is greater than or equal to a certain value, conversion of raw material is stable, then influence of external diffusion is considered to be excluded.
It was found that catalyticperformance was the best when Zn content was 2.54%. Therefore, Zn-HZSM-5 zeolite (Zn mass content of 2.54%) catalyst was chosen for MTG intrinsic kinetics study. Under the reaction conditions of pressure 1 MPa, temperature (673-723) K, space time (0.11-0.41) h and airflow velocity 0.10m· s-1, gas methanol concentration of 9.38%, the orthogonal experiment of methanol to gasoline was carried out.
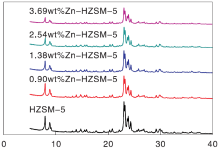
ZSM-5 zeolite catalyst has characteristic diffraction peaks at diffraction angles of 8.0° , 8.9° , 23.10° , 23.30° , 23.90° and 24.4° (JCPDS00-037-0359). XRD patterns of samples are showed in Figure 1. We can see from Figure 1 that five zeolite catalysts synthesized have the same crystalline structure as ZSM-5 zeolite and no other miscellaneous crystals appear. According to literature[23, 24], the intensity of diffraction peak at diffraction angle of 22.0° -25.0° can represent the crystallinity of ZSM-5 zeolite catalyst.In figure 1, HZSM-5 zeolite catalyst has the highest crystallinity and Zn-HZSM-5 zeolite catalyst with Zn mass fraction of 3.69% has the least poor crystallinity. With the increase of Zn content in Zn-HZSM-5 zeolite catalysts, the crystallinity showed a decreasing trend due to Zn element into the zeolite framework during in-situ synthesis. The characteristic diffraction peaks of ZnO do not appear in figure 1, indicating that the distribution of Zn atoms in the zeolite catalyst framework synthesized in-situ is very good.
Pore structurecharacterize by N2 isothermal adsorption-desorption is showed in Table 1. As shown in Table 1, specific surface area, pore volume and average pore diameter of the HZSM-5 zeolite catalyst are larger than that of Zn-HZSM-5 zeolite catalyst. With the increase of Zn content, specific surface area, pore volume and average pore diameter of Zn-HZSM-5 zeolite catalysts decrease, probably because the atomic radius of Zn is larger than Si and Al. When Zn enters into the framework of zeolite through in-situ hydrothermal synthesis, the pore volume and pore size of zeolite decrease[24].
| Table 1 Pore structures of samples |
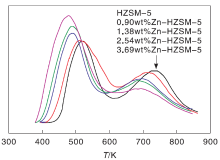
NH3-TPD characterization results are showed in figure 2. Weak acid has little effect on MTG reaction while strong acid have great influence. According to literature[25, 26], the desorption peak appearing in (423-573) K attributes to weak acid, the desorption peak appearing in (573-723) K attributes to medium strong acid, the desorption peak appearing in (723-823) K attributes to strong acid. As shown in Figure 2, Zn-HZSM-5 zeolite catalyst with Zn mass content of 3.69% has the lowest strong acid content and the highest weak acid content. HZSM-5 zeolite has the lowest weak acid content and the highest strongest acid content.With the increase of Zn loading in Zn-HZSM-5, the desorption peaks at low-temperature and high-temperature of Zn-HZSM-5 zeolites shift to left, and the contents of weak acids in Zn-HZSM-5 zeolites increase. At the same time, decreasing of the strong acid content indicates that the skeleton of Zn atoms on the zeolite catalyst surface acid ratio of three kinds of acid play a regulatory role[27].
When linear velocity of airflow is greater than a certain value, methanol conversion remains unchanged, and the impact of external diffusion can be ruled out. Linear velocity is determined by amount of zeolite catalyst and feed of methanol. The external diffusion experiments were carried out under reaction pressure of 1 MPa, reaction temperature of 673 K and space time of 0.21 h. The concentration of methanol in the operating gas is 9.38%.
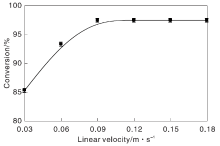
Effect oflinear velocity on methanol conversion is showed in Figure 3. As shown in Figure 3, when air stream linear velocity is gradually increased from the minimum value to 0.10 m· s-1, methanol conversion gradually increased. However, when air flow linear velocity is more than 0.10 m· s-1, methanol conversion keeps constant. Therefore, when linear velocity is greater than or equal to 0.10 m· s-1, the influence of external diffusion has been eliminated.
When zeolite catalyst particle size is smaller than a certain value, methanol conversion is independent of catalyst particle size, indicating that effect of internal diffusion is excluded. Continue to increase zeolite catalyst diameter, methanol conversion gradually decreases. The internal diffusion experiments were carried out under reaction pressure of 1 MPa, reaction temperature of 673 K, space time of 0.21 h, Zn-HZSM-5 zeolite catalyst loading of 1 g, average Zn-HZSM-5 zeolite size of 0.21 mm, 0.27 mm, 0.30 mm, 0.50 mm, 0.71 mm, 1.1 mm.
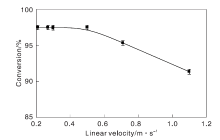
Effect of particle size ofZn-HZSM-5 zeolit on methanol conversion is showed in Figure 4. As shown in Figure 4, when Zn-HZSM-5 zeolite catalyst particle size is smaller than 0.30 mm, methanol conversion keeps constant, indicating that internal diffusion has been ruled out. Continue to increase Zn-HZSM-5 zeolite catalyst average particle size to lager than 0.30 mm, methanol conversion decreases. Therefore, when Zn-HZSM-5 zeolite catalyst particle size is or smaller than 0.30 mm, the inner diffusion has been eliminated.
Among factors affectingMTG reaction over Zn-HZSM-5 zeolite catalysts, temperature and space time are the main influencing factors. Therefore, in order to scientifically and efficiently study the influence of the two factors, an orthogonal experiment was used to design the experiment scheme. The intrinsic kinetics of MTG reaction over Zn-HZSM-5 zeolite catalyst (mass fraction of Zn 2.54%) was investigated free of internal and external diffusion. The orthogonal experimental was designed as reaction pressure of 1 MPa, reaction temperature of 673 K, 698 K, 723 K, space time of 0.11 h, 0.13 h, 0.16 h, 0.21 h, 0.33 h, 0.41 h. Results are showed in Table 2.
| Table 2 Kinetic data of methanol to gasoline |
In 1979, based on the carbene mechanism, Chen and Reagan established a lumped kinetic model which was used by later researchers to study the intrinsic kinetics of MTG reaction. Therefore, the kinetics of MTG reaction over Zn-HZSM-5 zeolite catalyst (Zn mass fraction of 2.54%) was also studied by the kinetic model established by Chen and Reagan. The three-lumped kinetic model is as follows:
(1) A (oxygenate) reacts to form C (lighter olefins). The reaction in this step is first order reaction. According to the Chen and Reagan kinetic models, methanol and dimethyl ether are oxygenate components and ethylene, propylene and butene are light olefins.
A$\xrightarrow{{k_{i}}}$C(1)
(2) The reaction of A with C to form C is an autocatalytic reaction.
A+C$\xrightarrow{{k_{i}}}$C(Autocatalytic step)(2)
(3)C can generate G (gasoline component) through hydrogen transfer, oligomerization, aromatization, etc. The reaction is also a first order reaction. Gasoline component contains C5-C11 hydrocarbons.
C$\xrightarrow{{k_{i}}}$G(3)
k1 is the reaction rate constant for oxygenates to light olefins; k2 is the reaction rate constan for oxygenates and light olefins to light olefins; k3 is the reaction rate constan for light olefins to gasoline.
The above three reaction rate constants and temperature are in line with the Arrhenius equation. The mass conservation equation of Xiis:
Where Xi represents the mass content of corresponding component,
The pre-exponential factork0i and activation energy Eai has given the initial value. According to Arrhenius equation, the reaction rate constant ki of each component is:
In the formula, ki represents the reaction rate constant of each component; k0i represents the pre-exponential factor of each component; Eai represents the apparent activation energy; T represents temperature.
Through the fourth-order Runge-Kutta method and the least-squares regressionof kinetic constants, OF value of the target residual function is minimized.
OF
In the formula,
The target residual function OF value indicates fitting result of experimental value to calculated value of the lumped model. The smaller OF value is, the better the fitting result is. According to literature, OF value is generally [0.270× 10-2, 0.275× 10-3], OF value for Zn-HZSM-5 zeolite catalyst kinematic target residual function is set to 0.412× 10-3.
In the case ofOF value is 0.412× 10-3, through fourth-order Runge-Kutta method and least squares regression of kinetic constants, simultaneous equations (5)-(7) and mass conservation equation (4) are solved. The initial values of pre-exponential factor k01-k03and activation energy Ea1-
| Table 3 Three lumped kinetic model constant regression results |
Bring numerical value of k0i and Eai into ki expression (8), then:
Formation rate of light olefin from oxygenate k1:
Formation rate of light olefin from oxygenate and the light olefin k2:
Formation rate ofgasoline from lower olefin k3:
k1 is the largest, k2 is slightly less, both of them are in the same magnitude. k3 is the smallest. According to ki, it can be concluded that it is very easy for oxygenate to light olefins on Zn-HZSM-5 zeolite. The formation rate of light olefin components is faster than that of gasoline components. The calculated activation energy Eai of each reaction is in the same magnitude. The correlation coefficients R2 of the three components are greater than 0.993, indicating that the experimental values of each component are in good agreement with the simulated values. The lumped kinetic model established by Chen and Reagan can well describe the reaction kinetics of MTG reaction over Zn-HZSM-5 zeolite catalysts.
Whetherthree-lumped kinetic model used is fit for reaction kinetics of MTG reaction over Zn-HZSM-5 zeolite catalyst is investigated. Comparison between experimental and simulated values based on the kinetic model established by Chen and Reagan is showed in Figure 5. As shown in Figure 5, at different temperatures and space time, experimental values of XA (oxygenate), XC (low carbon olefin) and XG (gasoline component) are in good agreement with simulated results of the lumped model, which shows that the three-lumped model established by Chen and Reagan is very suitable for the reaction kinetics of MTG reaction on Zn-HZSM-5 zeolite.
The experimental values of XA and XC slowly decrease with the increase of space time at 673 K, 698 K and 723 K. The experimental value of XG increases slowly with the increase of space time. Simulated and experimental values are completely consistent in changing trend, which shows that the simulation and the experimental data obtained by the three-lumped model established by Chen and Reagan are in good agreement.
(1) Zn-HZSM-5 zeolite catalyst was prepared by in-situ synthesis method by introducing Zn into the framework of ZSM-5 zeolite and characterized by XRD, BET and NH3-TPD.
(2) MTG activity of Zn-HZSM-5 zeolite catalysts was evaluated using miniature fixed bed adiabatic reactor. Results showed that Zn-HZSM-5 zeolite catalysts exhibited the best activity when Zn mass fraction is 2.54%.
(3)When reaction pressure was 1MPa, reaction temperature was (673-723) K, space time was (0.11-0.41) h, intrinsic kinetics of methanol-to-gasoline production on Zn-HZSM-5 (Zn mass fraction of 2.54%) zeolite catalysts was studied by orthogonal experiment.
(4) Based on three-lumped kinetic model established by Chen and Reagan, ki calculated by fourth-order Runge-Kutta method and least-squares regression were
(5)Correlation coefficients (R2) between the simulated and experimental values of all the components are greater than 0.993 when taking the target residual function (OF) as test value for three-lump dynamical model and fitting effect of the experimental value. At different reaction temperatures, with the increase of space time, the trend of experimental values of oxygenates, light olefins and gasoline is in agreement with simulated value. Three-lump kinetic model of methanol to gasoline established by Chen and Reagan can be used to describe the intrinsic kinetics of MTG reaction on Zn-HZSM-5 zeolite catalyst.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|