作者简介:张肖肖,1986年生,女,山东省泰安市人,博士,讲师,研究方向为环境催化。
氮氧化物是大气主要污染物之一,严重危害生态环境和人类健康。选择催化还原在富氧条件下能够高选择性的消除氮氧化物。氢气选择催化还原氮氧化物(H2-SCR)因低温高活性备受关注。综述H2-SCR研究中活性中间物种的研究进展,概述红外谱图中不同活性物种的吸收谱峰,对活性物种形式及所处位置进行总结和分类。
Nitrogen oxide (NOx) is one of the main pollutants in atmosphere,it seriously endangers ecological environment and human health.Selective catalytic reduction (SCR) is a technology that can selectively eliminate NOx in presence of excess oxygen.H2-SCR has attracted much attention due to its high activity at low temperature.This paper reviews the research progress of different kinds of active intermediate species and outlines the adsorption band of different active species in IR spectra.The form and site of active intermediate species are summarized and classified with an emphasis.
固定源(如电厂)废气中的氮氧化物(NOx)是大气主要污染物之一, 引发多种环境问题, 严重危害人类健康。选择催化还原(SCR)是工业上应用最广的脱硝技术之一, 在富氧气氛、催化剂存在条件下, 还原剂优先与废气中的NOx反应使其还原为N2。用于SCR的还原剂有NH3、烃类(HC)和H2等。与(300~400) ℃[1, 2, 3, 4]的NH3-SCR和(300~600) ℃的HC-SCR[5, 6, 7, 8, 9, 10, 11, 12]相比, 在贵金属上, H2可在(100~150) ℃活化还原NOx[13, 14, 15, 16, 17]。此外, H2清洁、无污染、价格低廉、供给方便, 汽车尾气和电厂废气中均含有可供利用的H2, H2-SCR技术有希望应用于汽车和电厂废气中NOx消除。
自1971年Jones J H等[18]首次研究Pt/Al2O3催化剂上H2-SCR反应以来, 人们对H2-SCR反应的催化剂类型[13, 19, 20]、催化剂预处理条件[19]、反应条件[21, 22]及反应机制[22]等方面进行了大量研究。还原反应机制主要有三种观点:(1)吸附生成NO型物种或NOδ +物种; (2)吸附生成亚硝酸盐和/或硝酸盐物种; (3)反应生成NHx物种、NOx、N
负载型贵金属催化剂上, 反应混合气中的NO以NO和/或NOδ +形式直接吸附在催化剂活性组分或载体上, 过程中发生如下反应:
NO(g) ⇌NOads
NOads→ Nads+Oads
NOads+Nads→ N2O
Nads+Nads→ N2
H2(g) ⇌2Hads
2Hads+Oads⇌H2O
负载型Pt催化剂上, 活性NO和NOδ +物种主要位于Pt位上, 且与Pt的存在状态和催化剂类型相关。
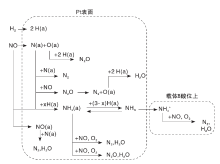
Burch R等[14]发现, Pt/SiO2催化剂上NOx吸附生成NO预吸附物种(NOpreads)、NO吸附物种(NOads)、NO二聚物[(NO)2和(NO
 | 图1 NO-H2-O2反应中Pt/SiO2催化剂上表面物种反应路径Figure 1 Reaction route of surface species on Pt/SiO2catalyst in NO-H2-O2 |
Frank B[23]和Marina O A[24]发现Pt表面NO是Pt-Mo-Co/α -Al2O3和Pt/β -Al2O3催化剂在NO-H2-O2反应中的活性中间物种。Shibata J等[25]发现, Pt表面NO是Pt/MFI催化剂在H2-SCR反应中的活性中间物种, 在红外谱图中峰位为1 844 cm-1和1 941 cm-1。Schott F J P等[17]发现与不同尺寸Pt颗粒键合的NO是Pt/ZrO2催化剂在H2-SCR反应中的活性中间物种, 红外谱图中峰位为1 720 cm-1、1 790 cm-1和1 860 cm-1。Wu等[26]发现Pt-NO是Pt/Si-MCM-41和Pt/Al-MCM-41催化剂在NO-H2-O2反应过程中的活性中间物种, Pt/Si-MCM-41上活性物位于Pt缺陷位(1 755 cm-1), Pt/Al-MCM-41上活性物位于Pt颗粒平台位(1 710 cm-1)。Zhang等[27]通过原位反应红外谱图证实, 金属态Pt位上的NO(Pt0-NO, 1 475 cm-1)是Pt-W/HZSM-5催化剂上的活性中间物种, Pt位上的硝酸盐(Pt-N
Macleod N等[29]发现, 在NO-H2-O2反应中, Pd/TiO2催化剂在反应温度范围内有两个NO最大转化率(110 ℃和170 ℃~240 ℃)。原位漫反射红外光谱研究结果表明, 低温下NO解离吸附, 解离生成的两个Nad结合生成N2, Nad与NO反应生成N2O, 吸附NO是活性中间物种, 该类物种有5种存在形式:NO-(1 152cm-1), Pd2+-NO(1 868 cm-1和1 819 cm-1), Pd+-NO(1 795 cm-1), Pd-NO-(1 693 cm-1)和Pd0-NO(1 733 cm-1)。Twagirashema I等[30]发现, Pd/LaCoO3催化剂在反应温度范围内有两个NO最大转化率, 反应温度低于200 ℃时, 金属Pd颗粒上, H2与NO直接反应, 仅金属态Pd表面NO是活性中间物种。Miller D D等[31]发现, 反应气中NO在Pd/Al2O3和Ag-Pd/Al2O3催化剂Pd位上的吸附形式有Pd0-NO(1 746 cm-1)和Pd+-NO(1 828 cm-1), Pd0-NO是催化剂在H2-SCR反应中的活性物种。Furfori S等[32, 33]指出钙钛矿型催化剂La0.8Sr0.2Fe0.9Pd0.1O3上吸附NO物种是活性中间物种, H2-SCR反应机理为:
NOad+NO→ N2O+Oad
2NOad→ 2Nad+O2
2NOad→ N2+2Oad
反应混合气中NO和催化剂活性组分上的吸附氧反应生成亚硝酸盐和/或硝酸盐, 该物种还原路径有:(1)活性组分上的吸附氢将其直接还原; (2)亚硝酸盐和/或硝酸盐转移并固定在载体上, 活性组分上的吸附氢溢流至载体上将其还原。活性物种位于活性组分和/或载体上, 取决于催化剂组成和类型。
Hamada S等[35, 36] 通过红外光谱证明, 占据Pt表面具有低氧化态的NOx吸附物种(N
在H2-SCR反应过程中, 活性硝酸盐物种位于催化剂的活性组分(如Pt, Pd)表面和/或载体上, 活性组分表面的硝酸盐来自NO氧化, 载体上的硝酸盐来自Pt表面吸附NO物种氧化转移或者载体晶格氧氧化。
Costa C N等[34, 38]通过漫反射红外光谱研究发现, 在H2-SCR反应中, Pt/SiO2催化剂表面物种有:Pt表面桥式NO物种、NOδ +、单齿硝酸盐、双齿硝酸盐和桥式硝酸盐; 载体表面亚硝酰基和双齿硝酸盐物种。Pt表面的亚硝酰基(Pt-NOδ +, 1 900 cm-1)和单齿硝酸盐(Pt-NO3, 1 480 cm-1)是该催化剂上的活性中间物种。活性单齿硝酸盐物种的生成路径为:
O2⇌2Oads
NOads+Oads→ NO2ads
NO2ads+Oads→ NO3ads
Machida M等[19]发现在H2-SCR反应中Pt/TiO2-ZrO2催化剂表面物种有双齿硝酸盐和NO2。Pt表面的硝酸盐(1 575 cm-1、1 280 cm-1和1 023 cm-1)是活性中间物种, 反应步骤为:
NO+1/2O2+Oad→ NO3ad
NO3ad+2H2→ 1/2N2+2H2O+Oad
NO3ad+3/2H2→ 1/2N2O+3/2H2O+Oad
Costa C N等[15, 34]发现, 在H2-SCR反应中, Pt/La0.5Ce0.5MnO3催化剂上NOx主要有八种形式:Pt表面桥式或弯曲NO、单齿硝酸盐和双齿硝酸盐, 载体上亚硝酰、螯和亚硝酸盐、N2O2n-1(n=1和2)、单齿硝酸盐和双齿硝酸盐。Pt表面桥式或者弯曲NO物种(1 700 cm-1), 载体上的亚硝酰基(M-NO+和M-N
Wu等[26]发现在H2-SCR反应中, 对于Pt/Si-MCM-41和Pt/Al-MCM-41催化剂, Pt表面NO, 载体表面自由硝酸盐(1 370 cm-1)、亚硝酸盐(1 420 cm-1)、单齿硝酸盐(1 545 cm-1和1 520 cm-1)、螯和硝酸盐(1 585 cm-1)和桥式硝酸盐(1 630 cm-1)是活性中间物种。吸附NO氧化生成亚硝酸盐或硝酸盐, 储存在载体上。
Costa C N等[38]发现在H2-SCR反应过程中, 复合氧化物MgO-CeO2负载Pt催化剂上, Pt表面的含氮物种有桥式或者弯曲NO(1 670 cm-1)、Pt-NOδ +(2 000 cm-1~1 900 cm-1)、双齿硝酸盐(1 620 cm-1)。活性中间物种仅位于载体上, MgO载体上的双齿(桥式)硝酸盐(1 540 cm-1)发生不可逆化学吸附; 与CeO2相邻金属阳离子-氧阴离子位上的硝酸盐(N
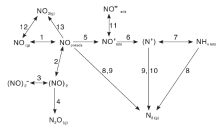
Itoh M等[39]发现载体上的硝酸盐(1 305 cm-1和1 466 cm-1)是Pt/CeO2催化剂在H2-SCR反应中的活性中间物种。其生成路径如图2所示。
 | 图2 H2-SCR反应中Pt/CeO2催化剂上NOx表面物种变化Figure 2 Transformation of NOx surface species over Pt/CeO2 in H2-SCR |
武鹏等[40]发现, 载体上的N
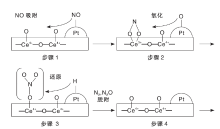
Machida M等[41]发现载体上的亚硝酸盐和硝酸盐是Pd/MnOx-CeO2催化剂在H2-SCR反应中的活性中间物种, 其存在形式为螯和亚硝酸盐(1 200 cm-1)、单齿硝酸盐(1 460 cm-1、1 300 cm-1和1 050 cm-1)和双齿硝酸盐(1 630 cm-1、1 550 cm-1、1 050 cm-1), 结构和生成路径如图3所示。
 | 图3 H2-SCR反应中Pd/MnOx-CeO2催化剂上活性中间物种结构和生成路径Figure 3 Structure and formation route of active intermediate species on Pd/MnOx-CeO2 in H2-SCR |
在H2-SCR反应中, Au/NaY和Au/ZSM-5催化剂[42]表面物种有NO-Au(1 780 cm-1)、NO-AuIO(1 760 cm-1), 固态N2O4(1 715 cm-1), 气相N2O4(1 704 cm-1), NO2和N
2NO+2Oads→ [2NO2⇌N2O4]ads
N2O4(NO2)+2H2→ N2+2H2O
2NO+2H2→ N2+2H2O
Shibata J等[25]发现, 在NO-H2-O2反应过程中, Pt/MFI催化剂载体B酸位上有N
Pt/Al-MCM-41[26]、Pt-W/HZSM-5[27]、Pt-Cr/ZSM-35[28]和Pt/HY[22]等催化剂上原位生成的N
| 表1 不同催化剂上活性NHx物种和N |
Qi G等[43]发现, 在H2-SCR反应过程中, Pd-V2O5/TiO2-Al2O3催化剂上表面物种有Pd0-NO(1 740 cm-1)、气相或者弱吸附的NO(1 904 cm-1和1 837 cm-1)、NO2(1 611 cm-1)、硝酸盐(1 583 cm-1、1 348 cm-1、1 300 cm-1)和N
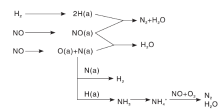 | 图5 H2-SCR反应中Pd-V2O5/TiO2-Al2O3催化剂上活性N |
Pd/Al2O3[44]和Pd/TiO2[29]催化剂上原位生成的N
(1)负载型Pt或Pd催化剂因低温高活性在H2-SCR反应中备受关注。反应过程中, 活性NOx中间物种有:NO型、NOδ +、亚硝酸盐、硝酸盐、NHx和N
(2)活性物种在红外谱图上谱峰位置不同。NO型和NOδ +源自反应气中NO的直接吸附, 主要位于活性组分位上。亚硝酸盐和硝酸盐是NO被活性组分表面氧和/或载体晶格氧氧化生成, 位于活性组分位和/或载体上。NHx和N
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|