作者简介:黄 蕾,1991年生,女,陕西省渭南市人,在读硕士研究生,研究方向为催化裂化催化剂。
考察磷元素对原位晶化催化剂抗镍性能的影响,借助等温N2吸附-脱附、IR、XRD、MAT等分析表征磷改性后的催化剂样品。在固定流化床装置上评价催化剂反应性能。结果表明,采用浸渍法将磷负载于催化剂上,并对催化剂进行一定量的镍污染后,随着磷的质量分数的增加,催化剂的微反活性降低,重油转化率下降,C5汽油收率降低,未表现出良好的抗镍性能。
Effects of phosphorus on in-situ crystallization resistance of nickel catalysts were investigated in details. The phosphorus-modified samples were characterized by N2 adsorption-desorption,IR,XRD and tested in fixed fluidized bed.The results showed that phosphorus was loaded on catalyst by impregnation.When catalyst was subjected to a certain amount of nickel contamination,as the mass fraction of phosphorus increased,specific surface area of the catalyst was no longer increased,micro-reaction activity of catalyst decreased,conversion of heavy oil decreased,and yield of C5 gasoline decreased,did not show good nickel resistance.
With the inferior quality of feedstock oil and the intensification of heavy metal such as nickel, vanadium, iron and copper, a higher resistance to heavy metal and heavy oil conversion performance is proposed for catalytic cracking catalysts. In-situ crystallization catalyst is a kind of catalytic cracking catalyst. It is prepared by special process using natural minerals, which has excellent heavy oil conversion and heavy metal resistance, and broad market applicationprospects[1].
In catalytic cracking reaction process, nickel has strong dehydrogenation activity, and promotes the polycondensation reaction of unsaturated hydrocarbons to coke, which increases hydrogen yield in the dry gas and seriously destroys cracking selectivity of FCC catalyst[2]. Further, dehydrogenation product blocks pores of catalyst, lowers surface area, and affects the cracking activity. At present, most of the catalytic cracking catalysts with nickel resistance are developed based on the principle of changing the molecular sieve structure of catalyst or changing physical structure of catalyst matrix[3, 4, 5].
Due to particular preparation process, in-situ crystallization catalyst has small molecular sieve grains, acid center is fully exposed, and pore structure of the matrix is developed, which makes it superior in heavy oil conversion and heavy metal resistance[6, 7, 8, 9]. In this paper, in order to enhance heavy metal resistance, broaden type of catalyst and meet market demand, in the preparation process of in-situ crystallization FCC catalyst, phosphorus was introduced by impregnation method. It was then contaminated with heavy metal nickel to study its resistance of nickel.
Main raw materials and reagents were showed in table 1.
| Table 1 Main raw materials and reagents |
Phosphorus was introduced to catalyst A by impregnation[4]. A certain amount of the catalyst was weighed, ammonium phosphate was used as the impregnating raw material, and mass fractions of impregnation solutions were 0.0%, 0.5%, 1.0%, and 1.5%, respectively. Modified catalysts A were labeled as A0, A1, A2, and A3. The sample was baked at 120 ℃ for 4 h and then calcined at 600 ℃ for 2 h.
The modified catalyst was subjected to a contamination test of heavy metal nickel.A certain amount of nickel nitrate solution was added to completely wet the catalyst. During the period, stirred regularly, then dried the contaminated catalyst sample at 120 ℃ for 4 h, stirred at regular intervals. After drying for 2 h at a certain temperature, the finished product was contaminated.
Specific surface area, pore volume and pore size distribution of the in-situ crystallization catalyst were determined by classical isothermal N2 adsorption-desorption using Autochem-2920 temperature programmed desorber and Micromeritics ASAP-3000 automatic physical adsorption instrument.
Surface acidity of the in-situ crystallization catalyst was measured using Brook-TENSOR 27 type infrared spectrometer manufactured by Bruker, Germany.
Crystallinity of the catalyst was measured by XRD diffraction using D/max-2000PC type X-ray diffractometer manufactured by Rigaku Corporation of Japan. Operating voltage was 40 kV, working current was 20 mA, CuKα radiation, phase scanning angle was 5° -50° , scanning speed was 10° · min-1. Crystallinity scanning angle was 22.5° -25.0° , scanning rate was 1° · min-1 .
Activity of the catalyst was determined in a fixed bed catalytic cracking micro-reactor adopting MAT type micro-reaction activity evaluation device produced by Beijing Huier Sanji Green Chemical Technology Co., Ltd., and the raw material oil was Tianjin Dagang straight-run light diesel oil.
Catalyst selectivity evaluation was carried out using a fixed fluidized bed XGL-2
| Table 2 Properties of raw material oil |
Table 3 shows texture properties of catalyst with different phosphorus contents.
| Table 3 Texture properties of catalysts with different P contents |
It can be seen from Table 3 that impregnation amount of phosphoric acid does not have a linear relationship with specific surface area, pore volume and pore size of the catalyst. Specific surface area of the catalyst gradually decreases as the amount of phosphorus increases. When phosphorus was not injected, specific surface area of the catalyst reached a maximum value of 305.5 m2· g-1. When phosphorus is loaded, specific surface area of the catalyst is smaller. Further, as the amount of phosphorus increases, pore diameter of the catalyst gradually increases. This is because the change of specific surface area of the catalyst causes change of pore diameter. The larger the specific surface area of the catalyst is, the smaller the pore diameter is.
It also can be seen from Table 3 that after phosphorus is supported on the catalyst, crystallinity of the catalyst does not change much. When the phosphorus content is 2.0%(A2), the change of relative crystallinity is 0, that is, there is no influence on crystallinity of the catalyst.
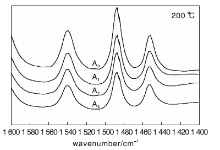
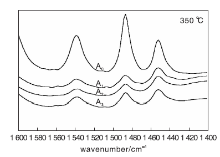
Surface acidity of the catalyst was determined by pyridine adsorption infrared spectroscopy. The results are shown in Figure 1 and Figure 2. Amount of B acid L acid obtained from Figure 1 and Figure 2 are shown in Table 4.
| Table 4 Acid quantity of catalyst with different P content |
B acid center is generally characterized by 1 540 cm-1 absorption band, and L acid center is characterized by 1 450 cm-1 absorption band. The total amount of B acid and L acid can be calculated from Lambert Beer’ s law. It is generally believed that the acid amount at 200 ℃ is the total acid amount, the acid amount at 350 ℃ is the strong acid amount and medium strong acid amount. The strong acid and medium strong acid center are cracking active center of the catalyst[4]. For the catalytic cracking catalyst, B acid is mainly provided by molecular sieve, L acid is mainly provided by matrix and binder. B acid guides positive carbon ion reaction, and strong B acid center helps to enhance the cracking ability of the catalyst for heavy oil. L acid mainly guides the reaction of positive carbon ions, and can also guide free radical reaction. Strong L acid is more likely to generate coke and dry gas. Weak L acid helps cracking of heavy oil macromolecules. Therefore, it is required that the catalyst has a proper amount of weak L acid center and tries to reduce the strong L acid center.
It can be seen from Table 4 that when phosphorus content is A0, the total acid amount, strong acid amount, medium strong acid amount and B/L value reach the maximum value. With the increase of phosphorus content, L acid amount decreases rapidly, amount of B acid reduced slowly, so that the B/L value is continuously reduced. This indicates that the addition of phosphorus does not enhance acidic active sites on surface of the in-situ crystallization FCC catalyst.
Whenphosphorus is loaded, total acid amount and strong acid amount decrease, which maybe due to the interaction of phosphorus with substance in the internal of the catalyst, thereby reducing the number of active centers on the catalyst surface. The amount of acid is reduced.
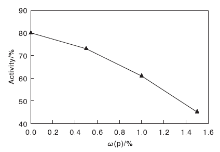
Using 0.5% nickel solution as the pollution source, samples A0 (0%), A1 (0.5%), A2 (1.0%) and A3 (1.5%) were contaminated, and the obtained products were respectively labeled as B0 (0%), B1 (0.5%), B2 (1.0%), B3 (1.5%). The micro-reaction activity values of B0, B1, B2, and B3 were experimentally determined and showed in Figure 3.
It can be seen from Figure 3 that the micro-reaction activity is the highest when phosphorus is not loaded, reaching 80%, and the micro-reaction activity decreases as phosphorus contents increases. When phosphorus content is B3(1.5%), the micro-reaction activity of the catalyst is reduced by 36% compared to B0. This indicates that when large amount of phosphorus is supported and the catalyst is contaminated with a certain amount of nickel, the micro-reaction activity of the catalyst is seriously lowered, which is disadvantageous for the catalytic cracking reaction.
The catalytic reaction performance of B0, B1, B2, and B3 catalysts was evaluated by fixed fluidized bed, and the results are showed in Table 5.
| Table 5 Catalytic performance of B0, B1, B2, and B3catalysts |
It can be seen from Table 5 that under the condition of nickel pollution, over B0 (0%) catalyst, conversion of heavy oil, yield of C5 gasoline, dry gas yield and liquefied gas are highest, yield of diesel, heavy oil yield and coke are lowest. This indicates that the cracking depth and cracking activity of the catalyst are strongest.
Under nickel polluted conditions, with the increase of phosphorus, heavy oil conversion and C5 gasoline yield decrease, yield of diesel and heavy oil increase. Products distribution is poor. This indicates that under the same nickel pollution conditions, loading of phosphorus on the catalyst can not improve conversion of heavy oil and yield of C5 gasoline, but the cracking depth is reduced, cracking activity and selectivity are weakened. At the same time, this also indicates that supporting phosphorus on the in-situ crystallization catalyst by impregnation method fails to improve nickel resistance of the catalyst. When phosphorus loading is B3 (1.5%), heavy oil conversion is greatly reduced, only 57.90%. This may be due to the excessive phosphorus blocking the catalyst pores, surface contact surface for heavy oil molecules and catalyst active is small, making the cracking reaction difficult
(1)Loading appropriate amount of phosphorus on the in-situ crystallization catalyst cannot increase its specific surface area and acidic active center;
(2) Under the condition of nickel pollution, with the increase of phosphorus loading on the catalyst, the micro-reaction activity of the catalyst decreases. Conversion of raw oil and yield of C5 gasoline decreases. Yield of diesel oil and heavy oil increase.
(3) Phosphorus on the catalyst introduced by impregnation fails to improve nickel resistance of the catalyst. Researchers may consider other methods of loading phosphorus onto the catalyst to improve nickel resistance.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|