作者简介:刘 欢,1990年生,男,博士,讲师,主要从事能源转化和C1化学方面的研究。
生物质作为重要的可再生绿色能源,由于资源丰富,分布广泛,S、N含量低,CO2零排放等优点,必将为实现可持续发展起到举足轻重的作用。乙醇作为一种最具潜能的汽油添加剂及化石燃料替代品,具有辛烷值高,无污染等优点。因此,催化生物质基合成气制乙醇作为可再生能源开发的途径之一,极具研究价值及工业价值。综述MoS2基催化剂上生物质基合成气制乙醇的重要研究进展,指出利用合成气催化制乙醇的效率还有待进一步提高, 尚未达到实际应用要求。改性MoS2基催化剂对乙醇选择性较高,但烃类及CO2副产物较多。为了促进乙醇的生成, 今后合成气催化制乙醇应深入系统的研究碱金属和过渡金属共同促进的MoS2基催化剂,并借助科学技术发展运用新方法和新思路, 制备高效、稳定的催化剂。
As clean and renewable non-fossil energy sources,biomass will play an important role in the sustainable development of energy because of widely distributed huge reserves,low contents of nitrogen and sulfur,and CO2 neutrality.As the most potential gasoline additive and alternative fuel for the replacement of fossil fuels,ethanol has the advantages of high octane number and no environmental pollution.Thus,heterogeneous catalytic synthesis of ethanol from biomass-derived syngas is a promising way for the development of renewable energy sources and of great research and commercial values.This paper briefly reviews the progresses and advances in the field of catalytic conversion of syngas to ethanol and points out that the efficiency of the catalytic conversion of syngas to ethanol is far from industrial application and needs to be further improved.Modified MoS2-based catalyst could enhance selectivity of ethanol,but also promote formation of hydrocarbons and CO2.In order to facilitate the production of ethanol,stable and efficient MoS2 based catalyst promoted by alkalis and transition metal should be studied systematically via new techniques and methods.
随着化石资源的不断消耗, 释放出大量的污染物, 全球气候不断恶化, 环境保护问题和能源短缺问题正受到世界各国的普遍关注。我国作为一个“ 贫油、贫气、富煤” 的国家, 煤炭资源丰富, 而石油与天然气资源储量较低[1], 煤炭的清洁利用显得尤为重要。此外, 生物质作为可再生资源, 是仅次于煤、石油、天然气排名第四的能源资源。我国生物质能资源相当丰富, 理论生物质能资源约有50亿吨标准煤, 是我国目前总能耗的4倍左右[2, 3, 4, 5]。气化和热解技术作为最具前景的能源利用技术之一, 通过气化和热解技术, 将煤和生物质资源转化为合成气有助于煤炭的清洁利用和生物质资源的大规模集中利用[6, 7, 8, 9]。
目前, 合成气主要用于制甲醇, 而乙醇比甲醇具有更高的附加价值, 除了作为燃料和汽油添加剂外[10, 11, 12, 13, 14, 15, 16, 17], 也是重要的化工原料, 用于生产其他化学品、燃料及聚合物, 如乙醇制醚、乙醇制丁醇、乙醇胺化反应、氧化反应、脱水制烃类物质和烷基化反应等[18, 19, 20]。近些年, 随着燃料电池的快速发展, 乙醇作为可再生氢能在燃料电池中的应用也得到了广泛关注和研究[21, 22]。因此, 催化煤和生物质基合成气加氢制乙醇, 具有重要的应用价值和市场前景。本文简要综述MoS2基催化剂上生物质基合成气制乙醇的重要研究进展, 并展望合成气催化制乙醇的未来发展前景。
未改性的MoS2基催化剂上CO加氢反应的产物只有烃类, 其中甲烷为主要产物。1984年, 美国Dow Chemical公司[23, 24]和Union Carbide公司[25]分别开发了一种新型碱金属改性MoS2为活性组分的催化剂, 用于催化合成气加氢制乙醇。反应生成的醇类主要为直链正构醇, 服从Anderson-Schulz-Flory分布。醇类和乙醇的选择性分别高达90%和30%。碱金属的掺杂促进了烃类向醇类的转化, 从而提高了醇类产物的选择性。MoS2基催化剂主要优势如下:(1)由于MoS2基催化剂自身含硫, 故具有较强的抗硫性。在反应过程中, 降低了催化剂被毒化的风险, 减少了生产成本; (2)催化剂表面不易积炭, 可在H2与CO物质的量比较低的情况下使用; (3)对CO2不敏感, 在反应过程中不必严格控制CO2的浓度; (4)有利于直链正构醇的生成, 且对乙醇选择性较高。基于上述等优势, 改性MoS2基催化剂一直被视为催化合成气加氢制乙醇最具应用前景的催化剂。
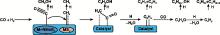
MoS2基催化剂上CO加氢制低碳醇的反应机理如图1所示[26, 27]。一般认为, 过渡金属以两种形式存在于改性的MoS2催化剂, 即硫化物形式和M-NMoS形式, 其中过渡金属的硫化物能够解离吸附CO, 而M-NMoS则以分子态吸附CO。H2和CO首先在催化剂表面吸附, CO在不同活性位以不同的形态吸附在催化剂表面。解离态的CO与H反应生成中间体甲基或亚甲基, 同时分子态的CO插入到C-M键形成烯醇中间体, 之后进一步加氢生成乙醇, 或加氢脱水生成C2+碳氢化合物中间体, 后再经过CO插入反应和加氢反应形成C2+醇类。
助剂类型对MoS2基催化剂的活性和醇类产物的分布及选择性具有重要影响。Liu Z等[28]在温度350 ℃、压力5.2 MPa、空速6 000 L· (kg· h)-1和H2与CO物质的量比为1的条件下, 利用固定床研究了碱金属改性MoS2基催化剂上合成气加氢反应的催化性能, 发现碱金属对催化性能的促进作用取决于碱金属和Mo的物质的量比。在物质的量比为0.7: 1时, K、Rb和Cs改性催化剂上C2~C4醇类产物的选择性分别约为42%、35%和40%。K和Rb的促进作用优于Cs, 且Cs的促进作用随着铯钼物质的量比的减少而增强。Muramatsu A等[29]研究表明, 在K修饰的MoS2基催化剂中, Mo4+是催化合成气加氢生成C2+醇类的活性组分, K的添加起到保护表面Mo4+作用, 防止其被还原为金属态Mo, 从而提高催化活性和C2+醇类的选择性。碱金属对钼基催化剂的促进作用顺序为Li< Na< Cs< Rb< K[30]。然而, 碱金属改性的钼基催化剂对醇类的选择性服从Anderson-Schulz-Flory分布, 限制了C2+醇类的生成。通过进一步添加过渡金属元素, 特别是Co和Ni, 可进一步提高催化活性和C2+醇类的选择性。Iranmahboob J等[31, 32]研究了K和Co改性的MoS2基催化剂对合成气加氢反应的催化性能, 结果表明, 这两种催化剂对乙醇均有较高的选择性, 其中K和Co共同改性的K-Co-MoS2/黏土催化剂上乙醇的产率高达130 mg· (gcat· h)-1。研究发现催化剂中同时存在Co3S4和Co9S8两种Co的硫化物, 其中Co9S8会导致催化剂活性降低, 且随着催化剂使用时间的增加而增加。此外, Co与Mo之间存在强烈相互作用, 并形成了Co-Mo-S结构, 在此结构中处于中间价态+3.5的Mo对CO加氢合成醇起到促进作用[33]。此外, K修饰的催化剂中Co9S8含量低于Cs促进的催化剂, 故K的促进作用优于Cs。Qi H J等[34]研究了两种助剂Ni和Mn对K/MoS2催化剂上合成气加氢反应的影响, 如表1所示, 从表1可以看出, 单独添加Ni能够促进C1到C2的同系化反应, 从而提高了催化剂的活性和对C2+醇类的选择性, 但Ni具有优良的加氢能力, 会加重反应过程中甲烷化反应, 生成大量烃类副产物[35]。而Mn具有抑制Ni在催化剂表面富集和提高Ni分散度的作用, 故Mn的引入大大抑制了Ni的加氢能力。在两种助剂的协同作用下, 催化剂的活性及对C2、C3醇的选择性得到明显提高。通过对Ni-K2CO3-MoS2催化剂的动力学研究, 发现Ni的引入能够降低
| 表1 Ni和Mn改性K/MoS2催化合成气加氢制乙醇的催化性能 Table 1 Catalytic performances of K/MoS2 catalyst modified by Ni and Mn for syngas hydrogenation to ethanol |
载体对MoS2催化剂上合成气加氢反应也有重要影响[38, 39, 40, 41, 42, 43, 44, 45, 46, 47]。通过对未负载和以Al2O3、SiO2、活性炭和多壁碳纳米管等为载体的MoS2催化剂催化合成气制乙醇进行研究, 结果见表2。由表2可知, 以活性炭和多壁碳纳米管为载体的催化剂具有更高的催化活性, 对C2+醇类有较高的产率和选择性。主要原因为Al2O3和SiO2为酸性氧化物, 不利于C2+醇类的合成, 而活性炭和多壁碳纳米管不仅具有惰性石墨表面, 减弱了与催化剂活性组分的相互作用, 从而使活性组分得到更加充分的利用, 而且还具有较大的比面积、合适的孔径分布和纳米级的孔道等特殊性质, 有利于活性组分在载体表面的高度分散, 增加活性表面。除受到助剂和载体的影响外, 催化剂制备方法对催化性能也有一定的影响[48, 49]。
| 表2 改性MoS2基催化剂上合成气加氢制乙醇的催化性能 Table 2 Catalytic performances of modified MoS2 based catalysts for syngas hydrogenation to ethanol |
随着化石能源的不断减少及世界各国对能源和环境问题的关注, 开发可再生资源和发展“ 绿色能源” 具有重要的战略意义。生物质作为重要的可再生绿色能源, 具有资源丰富, 分布广泛, S、N含量低, CO2零排放等优点。通过气化或热解技术, 可将生物质转化为合成气。采用合成气催化制乙醇的方法是一项极具挑战同时具有极大研究价值的工作。乙醇是优良的汽油添加剂和液体燃料, 乙醇的使用可有效减缓对化石能源的依赖, 同时减少污染物的排放。此外, 乙醇还是重要的化工原料之一。虽然经过大量的探索和研究, 同时取得了较大进展, 但利用合成气催化制乙醇的效率还有待进一步提高, 尚未达到实际应用的要求。改性MoS2基催化剂对乙醇选择性较高, 但烃类及CO2副产物较多。为了促进乙醇的生成, 今后合成气催化制乙醇深入系统的研究碱金属和过渡金属共同促进的MoS2基催化剂, 并借助科学技术发展运用新方法和新思路, 制备高效、稳定的催化剂。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|