作者简介:方志勇,1985年生,男,硕士,研究方向为工业催化。
含氯的挥发性有机化合物(chlorinated volatile organic compounds,CVOCs)应用广泛,但对大气环境污染严重。催化燃烧法具有转化率高,起燃温度低,运行能耗低以及副产物少等优势,是一种消除CVOCs的优势技术。催化燃烧处理CVOCs的催化剂可分为以Pt、Pd和Ru基为主的贵金属催化剂和以过渡金属为主的非贵金属催化剂。Pt、Pd基贵金属催化剂催化活性好,但价格高,对高温和氯中毒敏感。Ru基贵金属催化剂能催化Deacon 反应的发生,使Cl从催化剂表面脱除,提高了催化剂的稳定性。过渡金属复合氧化物价格相对低廉,抗中毒性能优良,但活性稍低于贵金属催化剂。还综述了CVOCs的反应、失活机理和再生方式,指出湿空气的加入能使催化剂再生,为催化剂的研究提供了参考。
Chlorinated volatile organic compounds (CVOCs) are widely used,but seriously pollute the atmosphere.Catalytic combustion has the advantages of high conversion,low ignition temperature,low operating energy consumption and few by-products.It is an advantageous technology to eliminate CVOCs.The catalysts for catalytic combustion treatment of CVOCs can be divided into noble metal catalysts based on Pt,Pd and Ru and non-precious metal catalysts based on transition metals.Pt and Pd-based noble metal catalysts have good catalytic activity,however,they are expensive and sensitive to high temp-erature and chlorine poisoning.Ru-based noble metal catalysts can catalyze the occurrence of Deacon reaction and improve the conversion and stability of the catalyst.Transition metal composite oxides are relatively inexpensive,which also have excellent catalytic and anti-poisoning performance for CVOCs,but the activity is lower than metal catalysts.This article also summarizes the reaction,deactivation mechanism and regeneration methods of CVOCs,and points out that the addition of humid air can regenerate the catalyst,which provides a reference for the research of the catalyst.
挥发性有机化合物(volatile organic compounds, VOCs)指在室温下饱和蒸气压高于70 Pa, 或常压下沸点低于260 ℃的有机化合物, 可导致雾霾、大气光化学烟雾、温室效应等一系列环境问题[1]。含氯的挥发性有机化合物CVOCs广泛应用于石油化工、制药及涂料等生产过程[2]。CVOCs毒性大, 直接排放至大气不仅污染环境, 还对人体造成伤害[3, 4]。由于CVOCs的高挥发性和稳定性, 使CVOCs的消除成为VOCs治理过程中的一个难题[5, 6]。
CVOCs的消除目前主要分为非破坏性的回收技术和破坏性的降解技术。非破坏性回收技术中的吸附法[7, 8]操作灵活, 成本低廉, 是一种适合处理低浓度CVOCs的高效方法; 吸收法、膜分离和冷凝法回收CVOCs能耗高, 价格昂贵[9]。破坏性降解技术中的光催化和生物降解虽然可以处理CVOCs, 但是需要的时间长, 能耗高, 而且副产物多氯代物选择性高, 因此实用性受到限制[10, 11]。高温燃烧(大于800 ℃)可以处理高浓度的CVOCs[12], 催化剂有助于在低温下进行降解, 节约能耗, 减少副产物的生成, 因此催化燃烧是一种较为适宜的CVOCs消除方式[13, 14]。
CVOCs催化燃烧催化剂分为贵金属和非贵金属催化剂。贵金属催化剂包括Pt基、Pd基和Ru基催化剂, 具有起燃温度低、活性高的优点, 但价格高, 资源缺乏, 且容易氯中毒; 非贵金属催化剂包括V、Ce、Cr和Mn基催化剂, 价格低, 抗中毒能力强, 但活性低于贵金属催化剂。因此, 需要提高过渡金属基催化剂的活性, 才能有一定的应用前景[13]。
本文综述近年来CVOCs催化燃烧的催化剂性能、催化反应/失活机理和再生方式, 指出湿空气的加入能使催化剂再生, 为催化剂的研究提供参考。
贵金属催化剂的研究主要集中在Pt、Pd和Ru上, Pt和Pd对C— C、C— H和C— O键具有较好的活化能力, 通过适当的负载方式, 将贵金属均匀分散在载体上, 对大部分VOCs均有较高的催化燃烧活性[15, 16]。然而, Cl在Pt和Pd表面吸附能高, 覆盖活性位, 使催化剂中毒。因此, Pt和Pd在催化燃烧CVOCs过程中抗毒性差, 且易产生多氯代副产物[17, 18, 19]。Ru基催化剂因其不易中毒, 活性较高且价格相对低廉, 广泛应用于CVOCs的催化燃烧过程中[20]。
Van Den BrinkRuud W等[21]将Pt负载在Al2O3上得到Pt/Al2O3催化剂, 研究其对氯苯(CB)催化燃烧的性能。结果表明, 在反应温度440 ℃时, 氯苯完全转化, 但产生大量的多氯代苯, 这是由于Pt 和Cl 生成的PtOCl物种催化氯苯进一步氯化生成多氯代苯。Scirè Salvatore等[22]将Al2O3载体换成H-ZSM-5和H-β 后, 催化燃烧氯苯的活性有所提高, 且沸石为载体时多代氯苯的选择性更低, 这是由于沸石的“ 择型效应” , 较小的沸石通道尺寸阻碍进一步氯化生成多代氯苯。
Matě jová Lenka等[23]采用溶胶-凝胶法制备了Ce0.5Zr0.5O2, 作为Pt和Au催化剂的载体。研究发现, 在Ce0.5Zr0.5O2载体上沉积Pt、Au可提高CeO2表面的还原性, 同时能降低催化剂的酸性, 提高了催化燃烧二氯甲烷(DCM)的反应活性。Topka Pavel等[24]将Pt和Au 负载在CeO2-ZrO2 复合金属氧化物上, 负载量为1%(质量分数)的Pt/CeO2-ZrO2催化氯苯完全氧化的活性最好。研究表明, 催化剂表面酸量及活性氧的数量均能影响催化剂的活性。
Giraudon J M等[25]将Pd负载在TiO2和ZrO2上, 发现Pd/TiO2催化氯苯燃烧的活性高于Pd/ZrO2, 这是由于TiO2还原性更好, 但两种催化剂均产生较多的多氯代苯。Gonzá lez-Velasco Jr等[26]制备了Pd负载量为0.1%~1.0%(质量分数)的Pd/Al2O3催化剂, 以1, 2-二氯乙烷(DCE)和三氯乙烯(TCE)为反应物, 发现负载量为0.42%(质量分数)的Pd/Al2O3的催化剂活性最好, DCE和TCE的完全转化温度分别为375 ℃和550 ℃。
Giraudon J M等[27]还研究了La基钙钛矿LaBO3(B=Co、Mn、Fe、Ni)负载Pd催化剂对氯苯催化燃烧性能。结果表明, Pd与钙钛矿之间存在协同作用, 促使Pd负载型催化剂活性高于纯钙钛矿, Pd晶粒尺寸越小, 越有利于Pd与LaFeO3之间的界面作用, 有利于氧空穴的更新, 从而提高催化活性。
含Ru的催化剂体系对CVOCs的催化燃烧效果很好, 转化率与稳定性都很高。主要原因是Ru能催化Deacon 反应的发生[28], 如式(1)所示, 吸附的Cl被O2氧化生成Cl2, 从催化剂吸附位点脱附, 能增强催化剂稳定性。载体一般选择本身具有催化活性、与催化剂之间作用较强的氧化物, 如Al2O3、CeO2和TiO2。
HCl+1/2O2=1/2Cl2+H2O (1)
1.3.1 Al2O3载体
Miranda Beatriz等[29]采用Al2O3载体制备了质量分数0.5%的Ru/Al2O3催化剂, 当质量空速WHSV=55 h-1时, TCE的完全转化温度为360 ℃, 副产物为四氯化碳和三氯甲烷。Yoon Wang Lai等[30]在Al2O3载体中掺杂Cr, 并研究了Ru负载量对催化TCE性能的影响。结果表明, Ru负载量为0.4%(质量分数)时可以提高催化活性。Ru形成了分散性好的Ru氧化物, 成为活化分子氧的活性中心并直接参与反应, 增强了催化剂的氧化活性, 促进向反应系统中供应活性氧。
1.3.2 TiO2载体
Cao Shuang等[31]在TiO2载体上负载贵金属Pt、Pd和Ru, 研究其对DCM的催化活性及稳定性, 结果如图1所示。由图1可知, Pt/TiO2活性及稳定性均较差; 与Pd/TiO2相比, Ru/TiO2催化剂的活性较好, 且在反应6 h后, DCM的转化率没有降低, 且副产物选择性没有明显提高, 表明Ru/TiO2催化剂稳定性更好。这是由于在Ru/TiO2催化剂上, DCM分解产生的C和Cl物种能迅速移除, 避免了积炭和中毒。
 | 图1 TiO2负载不同贵金属催化剂性能Figure 1 Catalytic performance of different noble metal catalysts supported by TiO2 |
Ran Le等[32]利用湿法浸渍法制备了质量分数1.0%的Ru/TiO2和Ru/Al2O3催化剂, 当反应原料DCM的体积空速GHSV=55 h-1, 反应温度235 ℃和267 ℃时, 1.0%Ru/TiO2催化剂的DCM转化率分别为50%和90%, 高于1.0%Ru/Al2O3催化剂。
Wang Jian等[33]通过湿法浸渍法在不同类型的TiO2载体(锐钛矿、P25和金红石)上负载Ru。结果表明, Ru在锐钛矿相上不稳定, 而在金红石相上非常稳定, 在P25上有最好的催化活性, TCE的完全转化温度为(260~270) ℃。Wang Chao等[34]发现负载型 Ru/TiO2的催化反应活性高于掺杂型 Ru-TiO2 催化剂, 这是由于钴氧化物与和 Ru 物种之间的相互作用提高了钌氧化物在钴氧化物表面的分散性, 从而提高了催化剂的活性和稳定性, 还可以降低氯化副产物的生成, 提高 HCl 的选择性。
1.3.3 CeO2载体
CeO2具有优异的储氧-释放氧能力, 且资源丰富, 是一种良好的催化燃烧催化剂载体, 是目前CVOCs的研究热点。
Huang Hao等[35]将Ru负载在不同形貌的CeO2载体上, 分别为棒状、立方块状和八面体状。结果表明, 不同晶面结构是影响Ru与CeO2载体之间相互作用的关键因素, 由{110}和{100}面包围的棒状CeO2最有利于Ru的活化和稳定, 形成大量的Ru-O-Ce键, 增加了Ru4+的含量和表面氧迁移率, 使氯苯氧化的活化能降低。这些结果有助于分析负载在不同形状/晶面CeO2的金属催化剂在CVOC氧化中的行为及开发高活性催化剂体系。
Dai Qiguang等[36]研究了掺杂Ti的CeO2负载催化剂(Ru/Ti-CeO2)对CB、DCM和TCE的催化活性, 并研究了Ti含量、Ru含量、反应物浓度、空速、氧浓度和水的影响。还探讨了其他金属, 如Mn, Co, Sn和Mg掺杂以及不同贵金属(Pt, Pd, Rh, Au和Ag)负载的影响。结果表明, Ti的掺杂可以明显提高CeO2基催化剂的催化活性和稳定性。
Dai Qiguang等[37]通过共沉淀制备了高比表面积的Ru/CeO2催化剂, 颗粒大小约7 nm, 在反应温度250 ℃时, 氯苯的转化率可达90%以上; Dai Qiguang等[38]还研究了氯苯在Ru/CeO2催化剂上的反应机理:氯苯中的C— Cl键在Ce3+/Ce4+活性位点上发生断裂, 当无氧气时, 形成中间产物苯; 当有氧气时, 解离的苯基可以被表面氧或晶格氧快速氧化成CO2和H2O。氯解离后吸附在活性位上, 导致催化剂快速失活。Ru/CeO2催化剂中的RuO2能催化Deacon 反应, 使解离吸附的Cl以Cl2的形式快速除去, 减缓催化剂的失活。
非贵金属催化剂价格相对较低、资源丰富且活性良好, 是一种潜在的替代贵金属CVOCs催化燃烧催化剂。常用于CVOCs催化燃烧的非贵金属催化剂主要是过渡金属氧化物, 如V基、Ce基、Cr基、Mn基及其复合氧化物。
Cho Chul-Hoon等[39]研究了负载型过渡金属氧化物和含钒的多金属氧化物催化剂, 用于催化燃烧剧毒的多氯芳族污染物, 当TiO2载体上V负载量为5%(质量分数)时, 有最佳的反应活性和稳定性。Wu Meng等[40]在锐钛矿和金红石结构的TiO2载体上负载V得到VOx/TiO2催化剂。结果表明, 负载在锐钛矿上的VOx/TiO2催化剂有更高的V5+-O-V5+氧传递速率, 提高了活性氧的迁移速率, 同时削弱了Cl的吸附, 并增强了从催化剂表面去除Cl的能力。Wang Jian等[41]结合动力学和原位FT-IR实验研究了氯苯在V2O5/TiO2催化剂上的氧化行为, 发现负载量为质量分数3%~5%的V2O5/TiO2催化剂最适合氧化氯苯。
Yang Peng等[42]通过不同的制备方法合成了4种CrOx-CeO2混合氧化物催化剂, 并研究了其对DCM的催化活性。结果表明, 通过共沉淀和微乳液法制备的CeO2-CrOx催化剂有更高的催化活性, 且对CO2和HCl的选择性更高。Yang Peng等[43]还通过沉淀法制备了不同Ce/Zr物质的量比的CeO2-ZrO2-CrOx催化剂, 结果表明, 添加适量ZrO2显著提高了CeO2-CrOx催化剂对DCM催化燃烧活性。当Ce∶ Zr∶ Cr物质的量比为2∶ 2∶ 1时, 较小的Zr4+和Cr3+能嵌入CeO2晶格中形成固溶体, 使催化剂具有良好的稳定性。Tao Fei等[44]通过沉积-沉淀法在不同载体上制备了CeO2-ZrO2-CrOx催化剂。结果表明, 催化剂的活性与载体的酸度之间具有强烈的协同作用, 可促进DCM降解的催化活性, 不同载体的活性顺序为:HZSM-5> TiO2> Al2O3> SiO2。
表1总结了几种Ce基催化剂的催化燃烧活性, 在Ce基催化剂中掺杂适宜比例的Cu和Mn元素, 能有效地提高CVOCs活性, 在反应温度264 ℃(T95)时, CB的转化率可达95%。
| 表1 Ce基CVOCs催化燃烧催化剂 Table 1 Ce based catalysts for catalytic combustion of CVOCs |
Cr具有良好的耐氯稳定性, 且在CVOCs催化燃烧过程中有较高的活性, 因此, 对Cr基催化剂也多有研究。
Ma Ruihong等[6]采用沉积沉淀法制备了不同Cr含量的CrOx/Al2O3催化剂, 发现在Cr含量为18%(质量分数)时对CH2Cl2有最高的活性, 并在反应温度350 ℃时完全氧化。CrOx/Al2O3催化剂中同时存在高氧化态的Cr物种和结晶态的Cr2O3, 且催化剂中Cr物种的平均价态随Cr含量的降低而降低, 而CH2Cl2氧化反应速率随Cr平均价的增加而增加, 表明高氧化态的Cr可能是反应的活性中心。Yim Sung Dae等[49]研究了SiO2、活性炭、丝光沸石、MgO、TiO2和Al2O3负载的氧化铬催化剂对四氯乙烯(PCE)的催化燃烧活性, 发现高比表面积的TiO2和Al2O3载体对于PCE的活性更高。
Mn元素价格低、对环境无副作用, 且耐氯性能好, Mn4+空位有较多的晶格氧, 对CVOCs有较好的催化活性。Liu Yan等[50]将不同含量的Mn负载在TiO2、Al2O3和SiO2载体上, 发现质量分数1.9%MnOx/TiO2有最高的催化活性, 在反应温度400 ℃时能完全转化氯苯。
Belkouch Jamal等[51]采用湿法浸渍法制备了MnCuOx/TiO2负载型催化剂, 在反应温度350 ℃时氯苯转化率达到100%, 且无多代氯苯生成。He Chi等[52]采用均相共沉淀法合成了大比表面积的Cu-Mn-Ce-O复合材料, 其中Mn和Cu以萤石状结构进入CeO2基体, 并产生大量的氧空位, 大量高价Mn4+离子的存在促进了还原铜相的形成, 表现出优异的催化活性, 氯苯在反应温度255 ℃的转化率可达90%以上, CO2选择性大于99.5%。
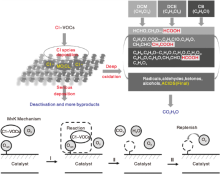
CVOCs催化降解涉及4个步骤:吸附、活化和Cl解离、C— Cl/C— C键断裂和深度氧化, 产物的解吸和脱附[53, 54, 55], 如图2所示。由于C— C和C— H键的键能大, CVOCs总是先发生Cl的解离[56]。如果解离出来的Cl能够及时脱除, 则完成催化循环; 如果不能及时脱除, Cl吸附在催化剂上, 导致催化剂中毒和副反应的增多。对于大多数催化剂, CVOCs的降解方式主要遵循Mars van Krevelen(MVK)机理[57, 58]:催化剂中的晶格氧将CVOCs氧化成CO2和H2O, 导致氧空位的形成, 通过吸附空气中的氧气补充氧空穴, 完成催化循环。
物理和化学原因都会引起CVOCs催化燃烧催化剂的失活, 如活性相的挥发、物理化学性质的改变、氯中毒、烧结和积炭[60, 61]。氯中毒、烧结和积炭减少活性位点的数量, 堵塞孔道并降低催化剂的表面积[31], 由这些因素引起的催化剂失活可以通过热处理[62], 化学再生[63]或引入具有适当湿度的空气再生[61], 因形成挥发性的金属氯氧化物而导致的失活则是不可逆的[51]。
Gallastegi-Villa M等[61]研究了H-BEA分子筛在催化燃烧DCM和TCE过程中的失活与再生性能。通过“ 反应-再生-反应” 循环, 分析每一个周期中CVOCs转化率的变化、催化剂在反应前后的酸性强度、焦炭含量和氯含量的改变。研究发现, 湿空气比干空气更有利于催化剂的再生, 主要是因为在较高的温度下, 湿空气更有助于除焦和除氯, 能在每一个反应周期中完全恢复催化剂的酸性, 从而使催化剂的稳定性提高。
Bertinchamps Fabrice等[64]研究了在反应过程中加入H2O对催化剂催化燃烧氯苯性能产生的影响。结果表明, 水的加入影响VOx的还原性, 同时减少强酸位点数量, 虽然在一定程度上能提高稳定性, 但是也导致氯苯的转化率降低。Cen Wanglai等[65]也通过第一性原理的方法指出H2O的引入会与空气中的O2产生竞争吸附, 导致催化剂的活性降低。
催化燃烧CVOCs的过程主要产生CO2, H2O和HCl, 减少了有毒有害的含氯污染物的排放。随着催化燃烧技术的不断发展, 催化剂正在逐步涉及到各个方面, 如贵金属, 过渡金属复合氧化物。Pt、Pd基贵金属催化剂催化活性好, 但价格高, 多氯代副产物选择性高, 对高温敏感, 且容易发生氯中毒, 这些因素限制了Pt、Pd基贵金属的应用。Ru基贵金属催化剂能催化Deacon 反应的发生, 提高了催化剂的转化率和稳定性, 应用前景良好。过渡金属复合氧化物价格相对低廉, 抗中毒性能优良, 但对CVOCs催化燃烧的活性稍低于贵金属。开发出低温高活性, 多氯代物选择性低, 积炭少的过渡金属催化剂是主要任务。
CVOCs在催化燃烧的过程中, 不可避免地发生Cl和C的吸附, 导致催化剂中毒和积炭进而失活。失活的催化剂在一定温度下通入湿空气可以将催化剂再生, 但是循环次数有限。在反应过程中加入H2O能提高催化剂的稳定性, 但是降低催化剂的活性。在今后的研究中, 可以在反应过程中加入一定浓度的水蒸气, 在保证催化剂的稳定性的同时, 制备出低温高活性的催化剂, 对推广CVOCs催化燃烧催化剂的工业应用有重要意义。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|